Yes, there are some limitations to the types of materials that can be filled in
HPMC capsules. HPMC capsules are suitable for filling with a wide range of powder, pellet, and granule materials, but there are certain materials that may not be compatible.
For example, materials that are highly hygroscopic (attract and retain moisture) may not be suitable for filling in HPMC capsules, as they can cause the capsule to become soft and sticky. Similarly, materials that are highly oily or greasy may not be compatible with HPMC capsules, as they can cause the capsule to stick together or become discolored.
It is also important to note that HPMC capsules may not be suitable for filling with certain active ingredients that require specialized delivery systems or specific dissolution profiles. In these cases, alternative capsule materials or delivery methods may need to be considered.
It is always recommended to consult with a qualified capsule manufacturer or formulation expert to determine the suitability of HPMC capsules for a specific material or application. They can advise on any potential limitations and provide guidance on the best capsule material and formulation options for a given product.
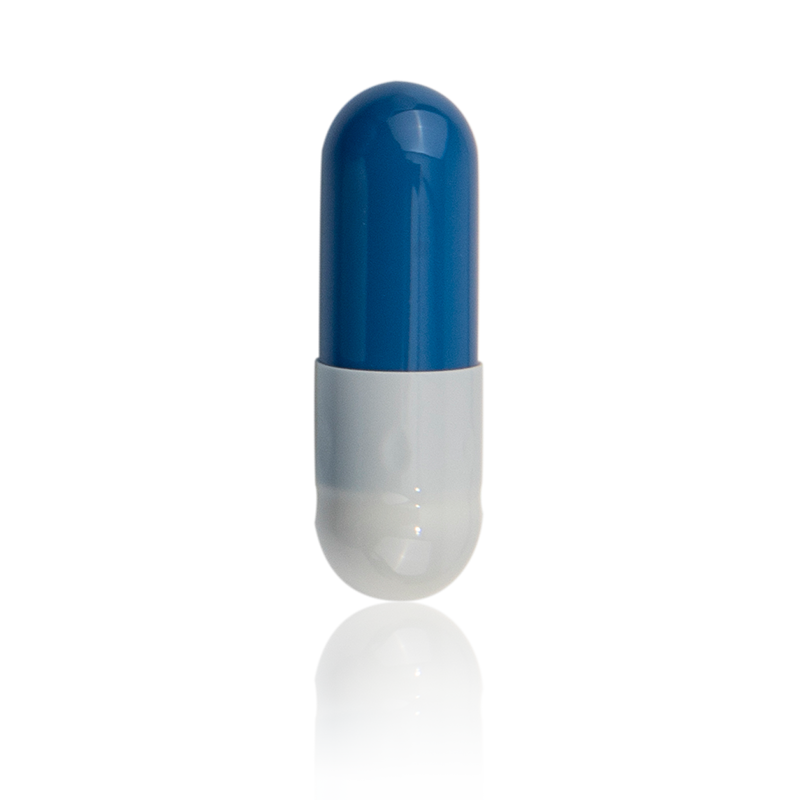
HPMC Hollow Capsules
Product introduction: The main raw material of hydroxypropyl methylcellulose hollow capsule is HPMC, which is extracted from natural cotton or wood pulp, which can effectively prevent foot-and-mouth disease.
Product Category: Pharmaceutical Excipients
Product application: HPMC has no side effects on the human body, and provides a new choice for vegetarians and people of different religious beliefs.
Product function: The drying weight loss of HPMC capsules is controlled within 6%, and the molecular structure is stable, and the moisture requirements of the filling are low. When filling drugs with strong hygroscopicity, the capsules will not be ruptured. It does not affect the moisture of the filler, making the filler safer and more thorough in dissolution.
Product advantages: its impurity residue is far lower than that of gelatin hollow capsules. Because it does not contain protein, coupled with the special production process, it is difficult for microorganisms to grow, so there is no need to add preservatives during the production process. After the product is finished production, the microbial limit meets the specified requirements, which can effectively prevent drugs and capsules from gelling. The combined reaction does not require additional sterilization.


 English
English Español
Español 中文简体
中文简体